Abstract
Introduction: Polycythemia vera (PV) is a chronic myeloproliferative neoplasm characterized by erythrocytosis. Thromboembolic events (TEs) are a common complication in patients (pts) with PV but can be reduced by controlling hematocrit (HCT) levels (<45%) with phlebotomy and/or hydroxyurea (HU), a first-line treatment (tx) for PV. However, in some pts HU can fail due to resistance and alternative tx options are needed, such as ruxolitinib. Machine learning was utilized to analyze real-world evidence (RWE) and identify clinical and/or laboratory variables that may be predictive of developing resistance to HU (HU-RES) tx.
Methods: PV-AIM (PV advanced integrated models for prevention of TEs) is an analytical, retrospective cohort study of pts with PV that utilizes data from the US Optum database. Pts were eligible for the current analysis if they had a diagnosis of PV, were ≥18 years old, had received only HU tx for 9 months (mo), had 6 mo pre-index (first HU tx) history (laboratory data and observations) and 12 mo post-index follow-up. An ensemble machine learning classification model, Random Forest (RF), was developed to predict HU-RES in the 6-9 mo window post-index, based on the 2010 European LeukemiaNet (ELN) consensus definition for HU-RES (criteria 1, 2 and 4), and evaluated by ROC-AUC (Receiver Operating Characteristic-Area Under the Curve). The 10 variables having the greatest impact on HU-RES prediction were identified using RF variable importance; those pairs of variables that showed greatest synergy (more significant association with HU-RES than expected) in predicting HU-RES were examined and a novel heatmap-based method was developed to visualize the risk landscape of each pairwise combination.
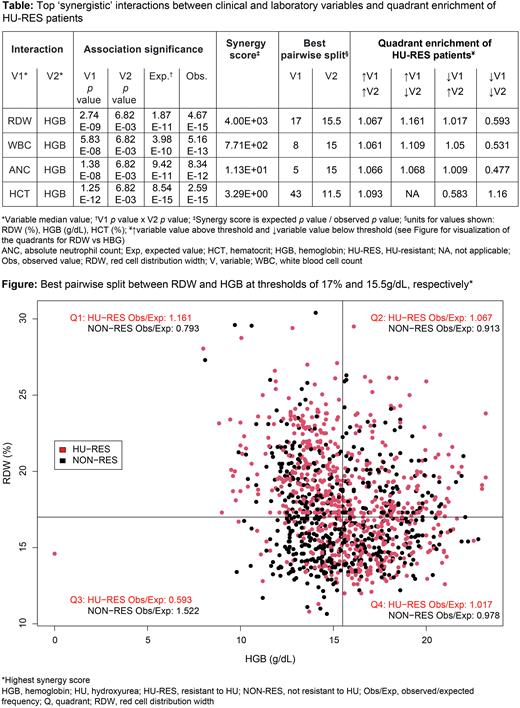
Results: Of 82,960 pts with PV identified in the Optum database (median record length 8.4 years), 2,453 pts were eligible for inclusion and 1,304 of these were assigned as HU-RES (n=733) or HU non-resistant (n=571) and were used to develop the model. The composite ROC-AUC was 0.71, illustrating the model's accuracy for predicting HU-RES at 6-9 mo post-index. Of the variables analyzed, the 10 most predictive for HU-RES (in rank order of importance) were: red blood cell count (RBC), hematocrit (HCT), red cell distribution width (RDW), age at index, hemoglobin (HGB), annualized phlebotomy count (APC), absolute neutrophil count (ANC), white blood cell count (WBC), weight, and time between diagnosis and treatment. These variables had higher values in the HU-RES pts except for 'age at index', which was lower in HU-RES pts. For these 10 variables, 45 pairwise combinations were generated to examine variable interaction. The HCT-APC pairwise combination had the strongest association with HU-RES (p=1.74E-22) at thresholds HCT 44% and APC 0.7; notably, APC was a key variable for the next 5 out of 10 most significant pairwise associations (in combination with RBC, ANC, WBC, RDW, and weight, respectively; p=7.16E-17 to 4.26E-22). Some combinations were synergistic; the most significant was RDW-HGB (synergy score: 4001) [Table] with optimal thresholds for this combination of RDW 17% and HGB 15.5 g/dL (p=4.67E-15) [Figure]. Comparisons of pts in the 'high RDW’ (≥17%; n=559) and 'low RDW’ (<17%; n=745) groups saw significant differences between these groups in WBC, ANC, RBC, lymphocytes, neutrophils, and HGB (p≤2.72E-09 for all), and neutrophil lymphocyte ratio (p=2.75E-37). Notably, a 1.36-fold greater proportion of HU-RES pts were in the 'high RDW’ than the 'low RDW’ group (p=2.74E-09).
Conclusions: Failure of HU tx may put pts at risk of TEs; however, this analysis has shown that common clinical and laboratory variables can be utilized to predict the likelihood of pts developing resistance to HU before starting tx. Pre-index HCT and APC were strongly associated with HU-RES and a synergistic association between RDW and HGB provided thresholds indicative of HU-RES, which potentially could be applied in the clinic. This analysis highlights the clinical value of pt history to help identify pts at risk of HU-RES and potentially empowers physicians to proactively schedule laboratory analyses pre-HU tx to identify pts at risk, and then closer, focused monitoring of at-risk pts to take timely clinical decisions for a switch to appropriate second-line therapy.
Disclosures
Verstovsek:Genentech: Research Funding; Constellation Pharmaceuticals: Consultancy; Incyte: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; NS Pharma: Research Funding; PharmaEssentia: Research Funding; Promedior: Research Funding; Protagonist Therapeutics: Research Funding; Roche: Research Funding; Sierra Oncology: Consultancy, Research Funding; Gilead: Research Funding; CTI BioPharma Corp.: Research Funding; AstraZeneca: Research Funding; Blueprints Medicines Corp.: Research Funding; Celgene: Consultancy, Research Funding; ItalPharma: Research Funding; Pragmatist: Consultancy. Heidel:Novartis: Consultancy, Honoraria, Research Funding. De Stefano:Abbvie: Other, Speakers Bureau; AOP Health: Other, Speakers Bureau; Novartis: Other, Speakers Bureau. Zuurman:Novartis Pharma AG: Current Employment. Bryan:Novartis Ireland Ltd: Current Employment. Afsharinejad:Novartis Ireland Limited: Current Employment. Kiladjian:Novartis, BMS, Incyte, AOP: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal